hRI Working Group
| Implementation | Publications | Presentations |
| Lipid Subcommittee | hCAMI Subcommittee | Resources |
Introduction
The CSCC Harmonized Reference Intervals (hRI) Working Group (CSCC hRI WG) aims to develop and implement evidence-based recommendations for pediatric, adult, and geriatric biochemical hRIs and test reporting across Canada. Since 2015, the CSCC hRI WG has applied evidence-based statistical analysis to determine indirect hRIs from ~300,000 – 13,000,000 datapoints per analyte obtained from community and hospital laboratories. Additionally, the CSCC hRI WG has provided key recommendations on the reporting of national clinical guidelines, including dyslipidemia reporting. The adoption of hRIs nationally is critical to improve and standardize the interpretation of laboratory test results and imperative to create cohesion between test providers.
Key objectives of The CSCC hRI WG include:
- Standardizing Reference Intervals – Developing and aligning reference intervals for common clinical laboratory tests.
- Promoting Evidence-Based Practices – Using robust methodologies and population-based data.
- Enhancing Quality & Patient Safety – Reducing variability in test interpretation.
- Collaborating with Stakeholders – Engaging health agencies, professional societies, and laboratory networks.
- Advancing Research & Knowledge Translation – Establishing evidence-based reference intervals and creating practical guidelines.
- Advocating for Global Harmonization – Contributing to international efforts and sharing best practices.
- Facilitating Education & Training – Providing resources to laboratory professionals and raising awareness among clinicians.
These efforts aim to enhance laboratory result reliability and improve healthcare outcomes in Canada.
Please contact us with any questions, suggestions, feedback, or speaking requests!
- Khosrow Adeli (khosrow.adeli@sickkids.ca)
- Christine Collier (christinecollier064@gmail.com)
Timeline of Key Deliverables
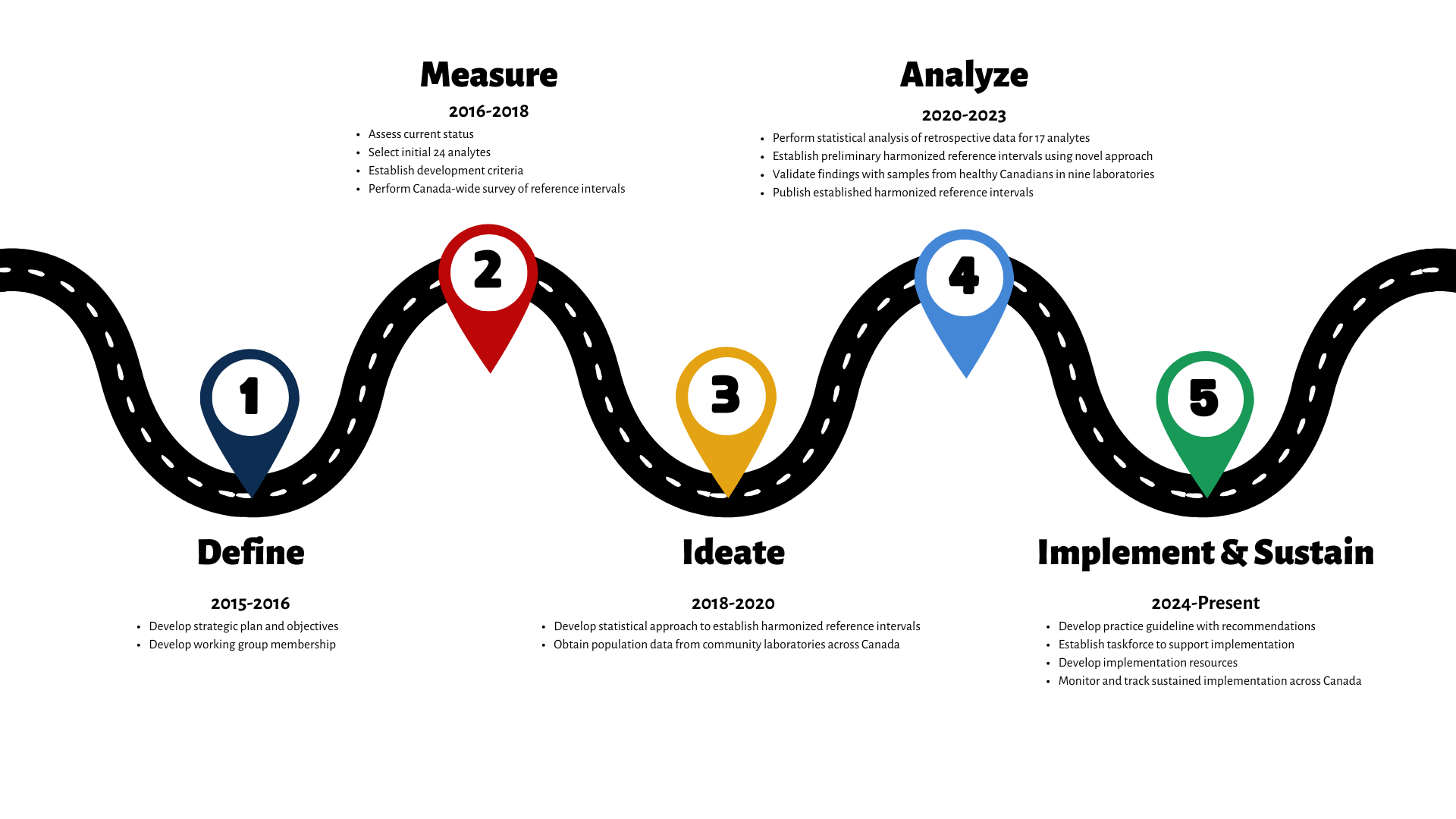
Ongoing Projects
Implementation – Phase I Analytes
We are working with laboratories to verify and implement harmonized reference intervals for Phase I analytes (alkaline phosphatase (ALP), alanine aminotransferase (ALT), albumin bromocresol Green (BCG), bilirubin total, calcium, carbon dioxide, chloride, creatinine, lactate dehydrogenase (LD), magnesium, phosphate, potassium, sodium, and thyroid-stimulating hormone (TSH)). See Implementation Page for more information.
Analysis – Phase II Analytes
The CSCC hRI WG is determining harmonized reference intervals for a second phase of analytes (including approximately 20 biochemical markers, 15 endocrine and fertility markers, and 15 hematology markers). The same indirect statistical approach will be used with verification of proposed hRIs using direct methods and verification in hospital sites.
Integrating guidelines into practice
Clinical guidelines are regularly published in areas of cardiovascular disease, diabetes, multiple sclerosis, endocrinology, and others. Although these guidelines provide clear instructions on how to screen for, treat, and monitor patients, they provide few details, if any, on how to appropriately present laboratory test results. To fill this gap and to ensure consistency in reporting across laboratories, the CSCC hRI WG regularly reviews clinical guidelines and provides recommendations on how to present laboratory reports.
- Harmonizing Lipid Reporting
The Lipids subcommittee was formed in 2018 with the goal of establishing recommendations for laboratory reporting of lipid tests in pediatric and adult patients to align with Canadian clinical guidelines. See the Lipid Harmonization Subcommittee Page for more information.
- Harmonizing CSF Analysis for MS Investigation
The Harmonized CSF Analysis for MS Investigation (hCAMI) subcommittee was formed in 2023 with the goal of establishing recommendations for laboratory processes and reporting of CSF oligoclonal banding and associated tests supporting MS diagnosis. See the Harmonized CSF Analysis for MS Investigation (hCAMI) Subcommittee Page for more information.
Upcoming Presentations
There are currently no planned upcoming presentations. Stay tuned for updates on where to hear from us!
Membership
| Co-Chairs | Khosrow Adeli Christine Collier |
| Members | Cynthia Balion Mary Kathryn Bohn George Cembrowski Victoria Higgins Benjamin Jung Zahraa Mohammed Ali Dana Nyholt Karina Rodriguez-Capote David Seccombe Jennifer Taher Albert Tsui Allison Venner Nicole White-Al Habeeb |
| Past Members/Other Contributors | Terence Agbor Berna Aslan Daniel Beriault David Blank Jake Cosme Anna Fuezery Angela Fung Trefor Higgins Josko Ivica Felix Leung Joe Macri Amber Marcano Pierre-Olivier Hetu Michelle Parker Isolde Seiden Long Janet Simons Julie Shaw Julia Stemp Vinita Thakur Dorothy Truong Uvaraj Uddayasankar Paul Yip |
| CSCC Head Office Support | Erica Lattimore |
| Academic Assistant | Olivia Landon |
| Representatives to Other Societies | Khosrow Adeli (ADLM, IFCC) Berna Aslan (IQMH) Mary Kathryn Bohn (TF-GRID) Joseph Macri (IFCC) Karina Rodriguez-Capote (IFCC cRIDL) Karen Scraba (BD) David Seccombe (ADLM) Isolde Seiden-Long (ISO) |
| Other Associations, Collaborators, and Consultants | Russell Price (President, Ontario Association of Pathologists –Barrie) Graham Jones (Australia) Ken Sikaris (Australia) Lina Santaguida (Epidemiologist (methodology), McMaster) Qing Fan (LifeLabs) |
| Lipid Harmonization Subcommittee Members | Co-Chairs: Victoria Higgins and Nicole White-Al Habeeb Khosrow Adeli Daniel Beriault Christine Collier Dana Nyholt Allison Venner |
| Harmonized CSF Analysis for MS Investigation (hCAMI) Subcommittee Members | Chairs: Victoria Higgins, Daniel Beriault and Michelle Parker Basma Ahmed Vipin Bhayana Ron Booth Yu Chen Christine Collier Mark Freedman Myriam Gagné Jessica Gifford Fabrizio Guiliani Ola Ismail Joe Macri Craig Moore Ashley Newbigging Lily Olayinka Ilia Poliakov Karina Rodriguez-Capote Raphael Schneider Simon Thebault Liju Yang |











